Emulsifier
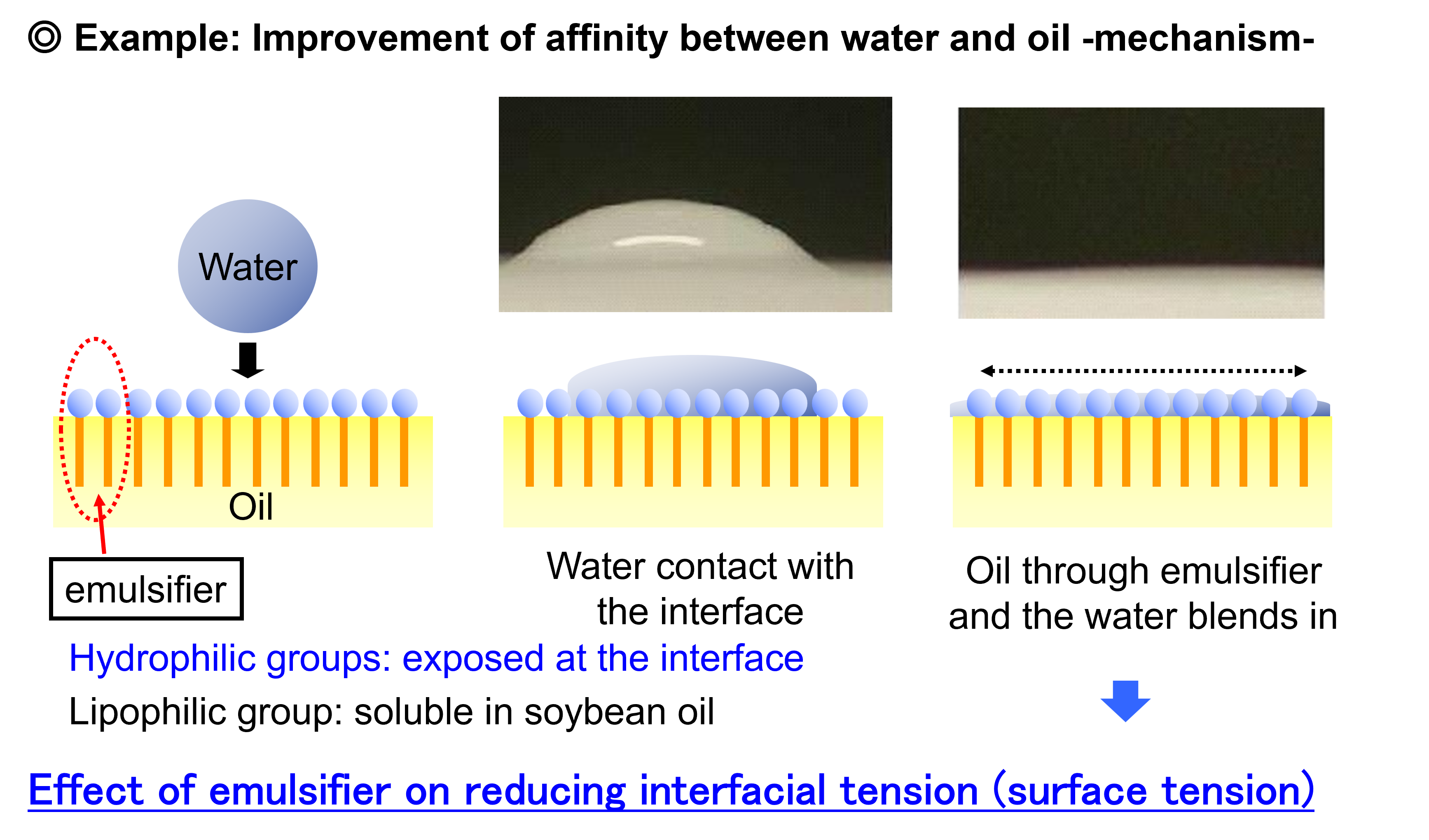
By adsorbing at the interface between water and oil and reducing interfacial tension, it facilitates the formation of fine particles, prevents coalescence, and maintains a stable state.
What is emulsification?
Emulsification is a key process in food production, enabling the stable blending of liquids that typically do not mix—such as oil and water. This process creates an emulsion, where one liquid is finely dispersed within another in the form of tiny droplets.
There are two main types of emulsions commonly used in food products:
- Oil-in-Water (O/W) Emulsions: Oil droplets are dispersed in water. Examples include milk and salad dressings.
- Water-in-Oil (W/O) Emulsions: Water droplets are dispersed in oil. Examples include butter and margarine.
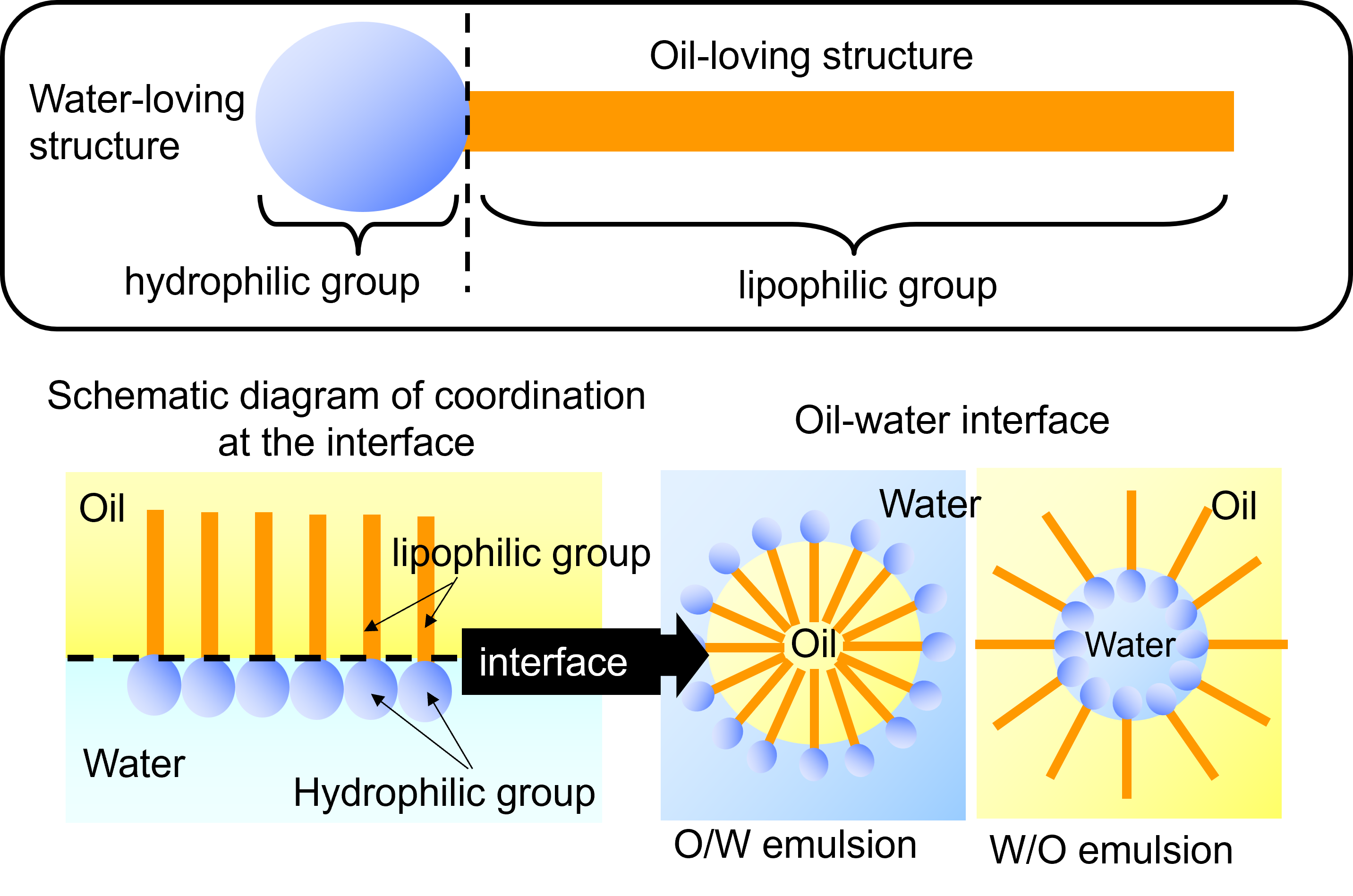
To maintain the stability of these emulsions, emulsifiers are used. Emulsifiers are compounds that possess both hydrophilic (water-loving) and hydrophobic (oil-loving) properties. Emulsifiers help stabilize emulsions by positioning themselves at the interface between oil and water, reducing surface tension and preventing the droplets from merging.
Structure and Function of Food Emulsifiers
Food emulsifiers are amphiphilic compounds, meaning they contain both hydrophilic and hydrophobic groups within their molecular structure. This unique dual nature allows emulsifiers to position themselves at the interface between immiscible liquids—such as oil and water—and reduce the interfacial tension.
By lowering this tension, emulsifiers enable the formation of stable emulsions, where:
- Oil-in-Water (O/W) emulsion:Oil droplets are finely dispersed in water.
- Water-in-Oil (W/O) emulsion:Water droplets are dispersed in oil.
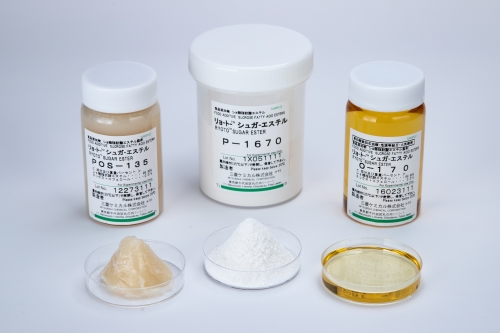
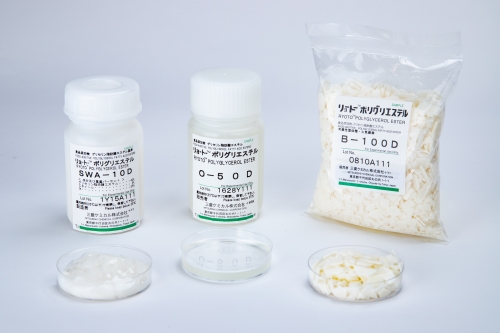
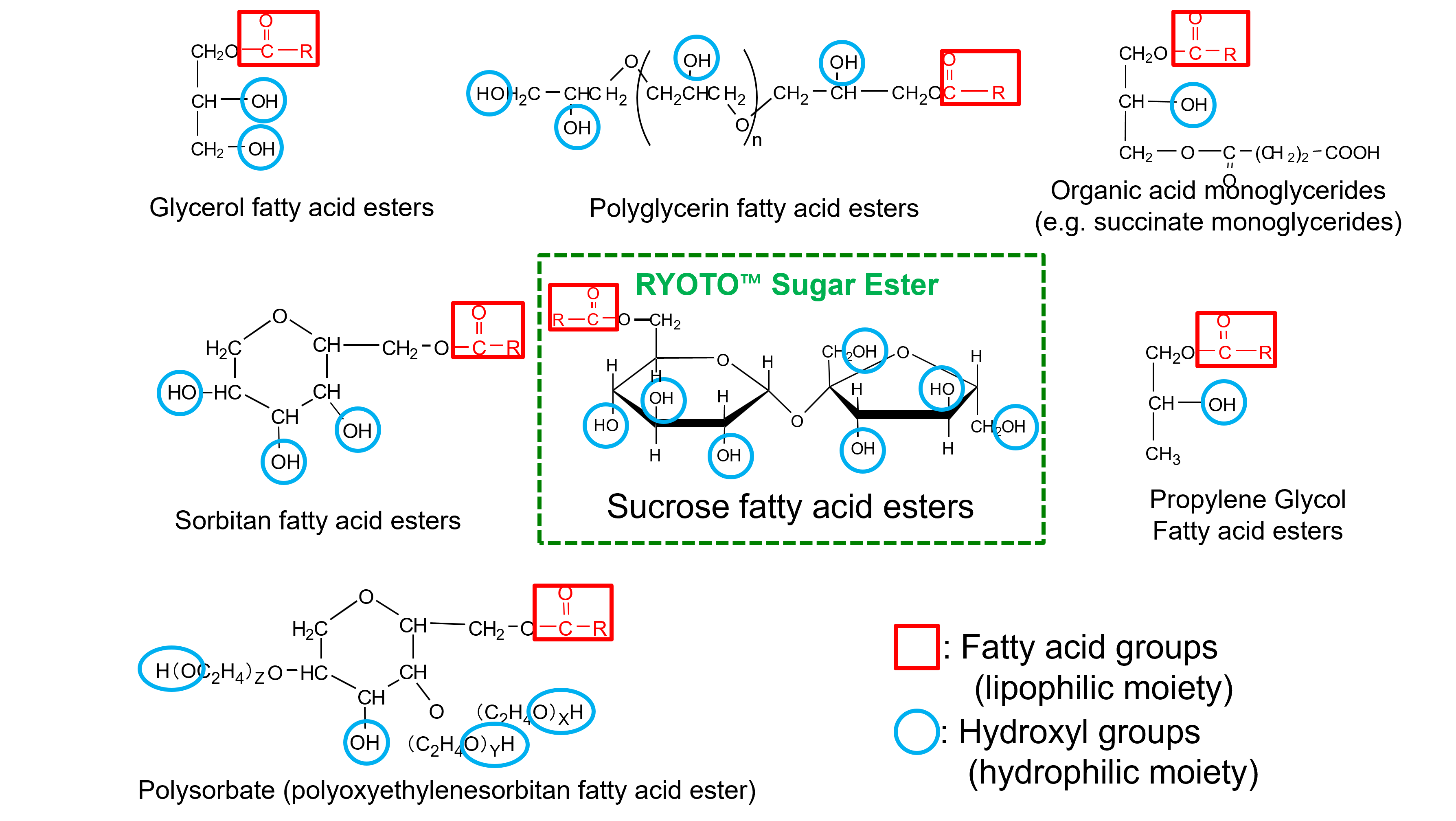
Types of Emulsifiers
(1) Types of Emulsifiers
In food manufacturing, a wide variety of emulsifiers are used, Each of them having distinct chemical structures and functional properties. Most food emulsifiers are composed of hydrophilic polyhydric alcohols and lipophilic fatty acids. The specific combination of these components—particularly the type of polyhydric alcohol—determines the characteristics and performance of the emulsifier.
Common Types of Emulsifiers are listed below:
-
- Sucrose fatty acid esters (Sugar esters)
- Polyglycerol fatty acid esters (Polyglycerol esters)
- Glycerol fatty acid esters (Monoglycerides)
- Sorbitan fatty acid esters
- Propylene glycol fatty acid esters
- Polysorbates
- Organic acid monoglycerides
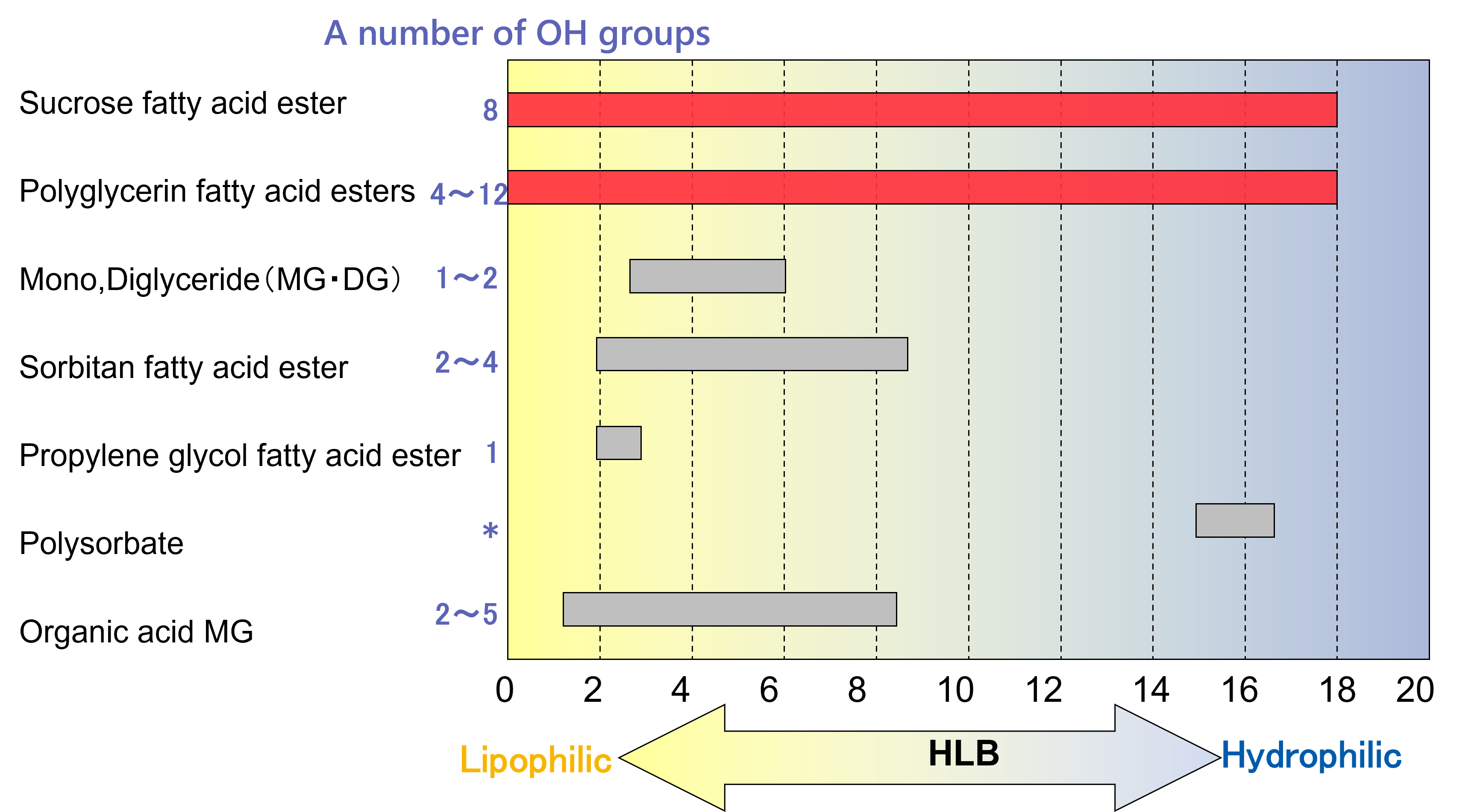
(2) HLB: Hydrophilic-Lipophilic Balance
The Hydrophilic-Lipophilic Balance (HLB) is a numerical scale used to describe the balance between hydrophilic and lipophilic properties of an emulsifier. HLB values range from 0 to 20, with lower values indicating greater lipophilicity and higher values indicating greater hydrophilicity.
This value serves as a practical guideline when selecting the appropriate emulsifier based on the desired type of emulsion.
Certain emulsifiers, such as sucrose fatty acid esters and polyglycerol fatty acid esters, offer a wide range of HLB values due to their molecular structures.
Sucrose esters can bind up to eight fatty acid chains, because sucrose have eight hydroxyl groups.
Polyglycerol esters can bind up to twelve fatty acid chains, because polyglycerin have appropriately twelve hydroxyl groups.
Characteristics and Applications of Food Emulsifiers
While the primary function of food emulsifiers is to stably disperse immiscible substances—such as oil and water—they also offer a wide range of additional benefits that enhance food quality and processing efficiency.
Thanks to their versatile properties, emulsifiers are used across many food categories. Key functions and applications include:
| Function Classification | Examples of Functions | Application Examples |
| Surface Activity | Reduction of surface tension | Cleaning of food products |
| Emulsion Stability | Stabilization of milk coffee emulsions | |
| Dispersion | Dispersion of calcium carbonate and titanium dioxide | |
| Foaming Ability | Improving volume of whipped cream and cakes | |
| Defoaming Ability | Preventing overflow in beverages, foam suppression during fermentation | |
| Solubilization | Clarification of added ingredients in beverages | |
| Effects on Starch (Complex Formation) | Prevention of retrogradation | Improving shelf life of bread, cookies, etc. |
| Delaying gelatinization | Enhancing the rise of wheat-based products when baking | |
| Reducing dough viscosity | Improving mechanical resistance of starch dough | |
| Effects on Proteins | Promotion of gluten formation | Improving quality of wheat-based products |
| Suppression of freezing denaturation | Enhancing freeze resistance of water-kneaded products | |
| Effects on Fats and Oils | Promotion or delay of crystallization | Improving freeze resistance of emulsions, preventing solidification of frying oils |
| Microcrystallization, Prevention of coarse crystallization | Bloom Retardation | |
| Reduction or increase of oil viscosity | Improving chocolate quality, dispersion of wheat components in fats | |
| Improvement of Powder Flowability | Prevention of caking | Prevention of caking, Improving flowability in powdered seasonings |
| Tableting and lubrication | Improving lubrication in tablet candies and pharmaceutical tablet |
Columns
We provide columns on industry trends and our products/technologies.
Various Downloadable Materials
Please download and view the related materials we have provided.